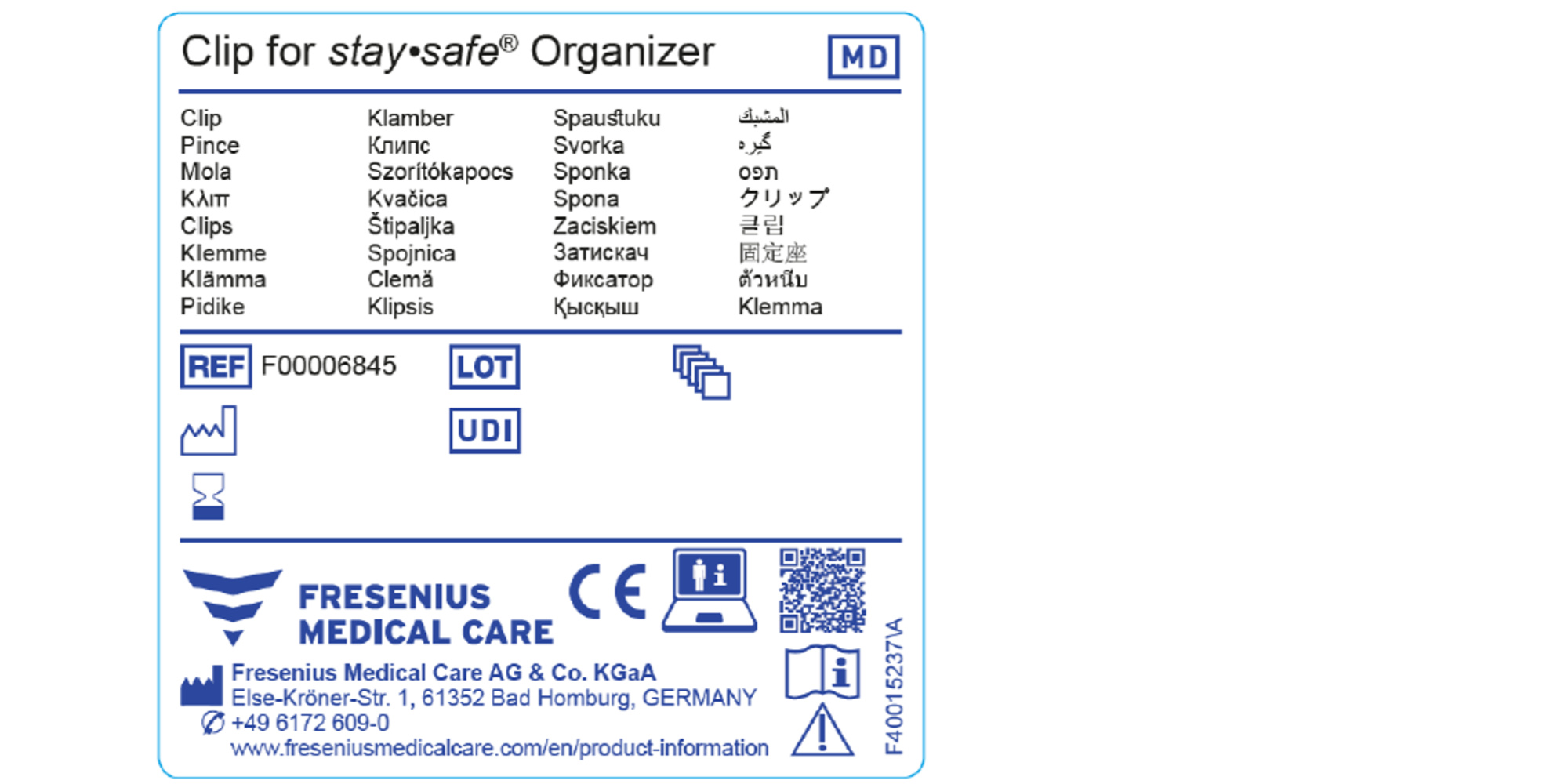
Eu Mdr Medical Device Labeling Requirements . Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no.
from www.freseniusmedicalcare.com
The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.
Medical Device Regulation Fresenius Medical Care
Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Eu Mdr Medical Device Labeling Requirements Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the. Eu Mdr Medical Device Labeling Requirements.
From operonstrategist.com
Traceability Requirements For Medical Devices in EUMDR Operon Strategist Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices. Eu Mdr Medical Device Labeling Requirements.
From www.presentationeze.com
EU Medical Device Regulation MDR 2017 745PresentationEZE Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.. Eu Mdr Medical Device Labeling Requirements.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. Regulation. Eu Mdr Medical Device Labeling Requirements.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Eu Mdr Medical Device Labeling Requirements The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.. Eu Mdr Medical Device Labeling Requirements.
From www.mantrasystems.co.uk
Achieve EU MDR medical device compliance Free Guide 2024 Eu Mdr Medical Device Labeling Requirements Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Regulation. Eu Mdr Medical Device Labeling Requirements.
From templates.rjuuc.edu.np
Medical Device Label Template Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label.. Eu Mdr Medical Device Labeling Requirements.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. The medical devices. Eu Mdr Medical Device Labeling Requirements.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label.. Eu Mdr Medical Device Labeling Requirements.
From gbu-taganskij.ru
EU MDR Medical Device Labeling Changes And Challenges By, 47 OFF Eu Mdr Medical Device Labeling Requirements The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Regulation. Eu Mdr Medical Device Labeling Requirements.
From meaningkosh.com
Mdr Medical Device Classifications MeaningKosh Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label.. Eu Mdr Medical Device Labeling Requirements.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the. Eu Mdr Medical Device Labeling Requirements.
From mdlaw.eu
MDR Checklist Labelling & IFU Requirements · MDlaw Information Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.. Eu Mdr Medical Device Labeling Requirements.
From mavink.com
Medical Device Labeling Symbols Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Manufacturers should include the name and trade name of the device and the manufacture date, if there is. Eu Mdr Medical Device Labeling Requirements.
From mavig.com
New Product Labeling due to MDR MAVIG Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label.. Eu Mdr Medical Device Labeling Requirements.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.. Eu Mdr Medical Device Labeling Requirements.
From www.microscan.com
Label Compliance and the New European Medical Device Regulations Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Manufacturers should include the name and trade name of the device and the manufacture date, if there is no. Regulation. Eu Mdr Medical Device Labeling Requirements.
From acf.com.tr
OEM PLM under MDR. Which model you will choose? Eu Mdr Medical Device Labeling Requirements Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Manufacturers should include the name and trade name of the device and the manufacture date, if there is. Eu Mdr Medical Device Labeling Requirements.